
Report ID: SQMIG35J2130

Report ID: SQMIG35J2130
sales@skyquestt.com
USA +1 351-333-4748

Report ID:
SQMIG35J2130 |
Region:
Global |
Published Date: May, 2025
Pages:
191
|Tables:
160
|Figures:
78



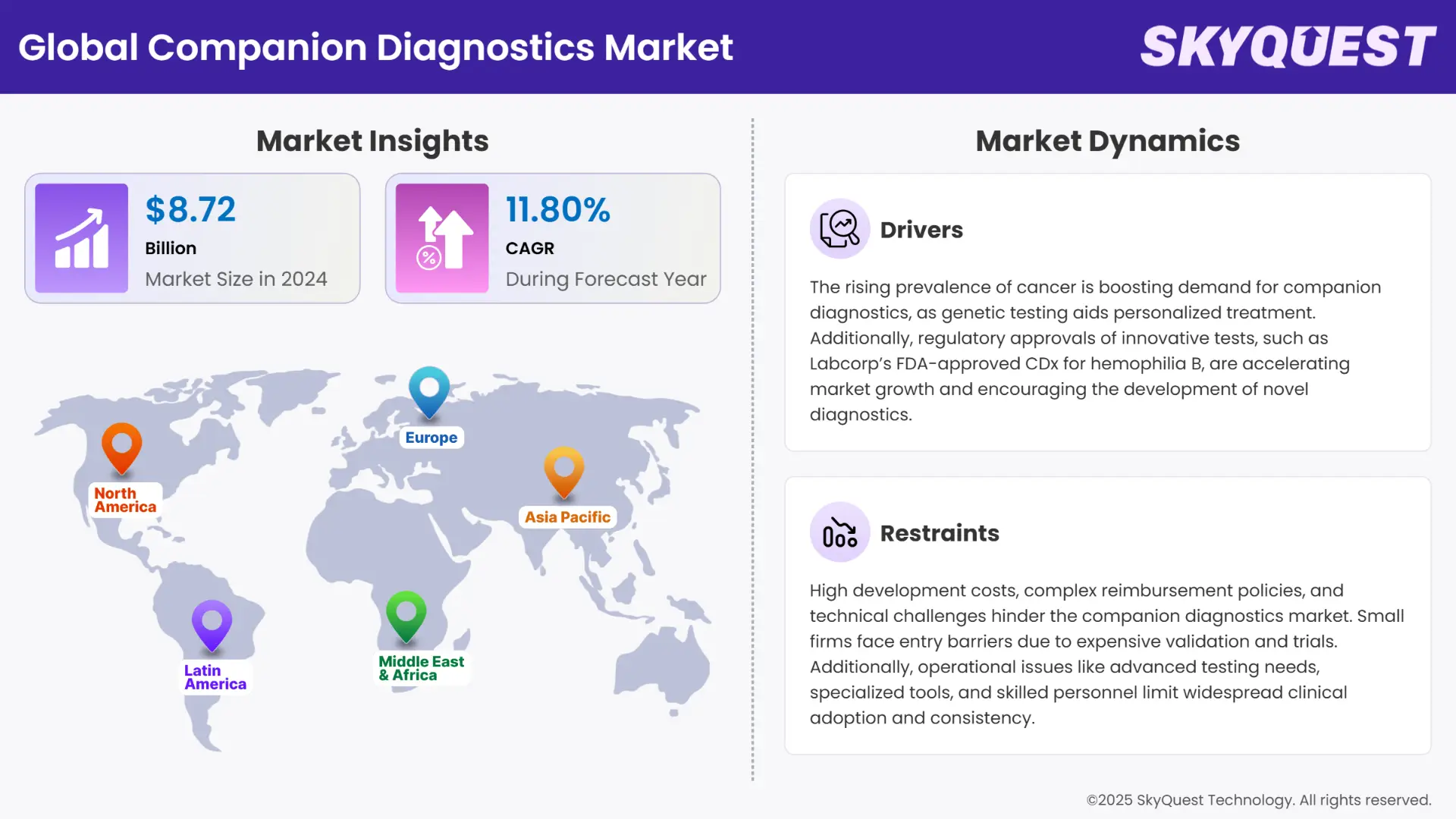
Global Companion Diagnostics market size was valued at USD 8.72 Billion in 2024 and is poised to grow from USD 9.75 Billion in 2025 to USD 23.8 Billion by 2033, growing at a CAGR of 11.8% in the forecast period (2026–2033).
The companion diagnostics market is experiencing quite a significant growth, driven by the increase in prevalence of cancer among global populations and technological advancements in genomic and molecular technologies. The market also finds itself in a suitable growing environment due to the rising regulatory support and the growth in demand among the masses for personalized medicine.
Bioinformatics and AI technology are being utilized in the market to accelerate biomarker discovery and improve the accuracy of diagnostics. Companion diagnostics is now not just confined to oncology. The market is witnessing increasing applications in other therapeutic areas such as neurology, cardiology, infectious diseases, and rare genetic conditions. However, the huge upfront costs associated with research, biomarker validation, clinical trials, and regulatory approvals for developing companion diagnostics remains a huge restraint for the market.
How is AI Technology bringing Transformation in the Market?
Artificial Intelligence (AI) technology is transforming companion diagnostics market dynamics. AI helps in advanced data analysis, image recognition, and predictive modeling. AI algorithms can help to analyze complex pathological images and genomic data. By doing this, they can identify biomarkers with more accuracy and speed. This is going to improve the precision of diagnostic and treatment decisions. Roche received FDA Breakthrough Device Designation for the VENTANA TROP2 RxDx Device in March 2025. This is the first AI-driven companion diagnostic for non-small cell lung cancer. This underlines the technology’s potential to bring about transformation in the companion diagnostics market globally.
To get more insights on this market click here to Request a Free Sample Report
The global companion diagnostics market is segmented into product & service, technology, indication, sample type, end user, and region. By product & service, the market is classified into assays, kits, and reagents, instruments & systems, and software & services. Depending on technology, it is divided into Polymerase Chain Reaction (PCR), In Situ Hybridization (ISH), Next-Generation Sequencing (NGS), Immunohistochemistry (IHC), Mass Spectrometry, Microarrays, and others. According to indication, the market is categorized into cancer (lung cancer, breast cancer, blood cancer, colorectal cancer, others), neurological diseases, cardiovascular diseases, infectious diseases, and others.
Depending on the sample type, it is divided into tissue samples, blood samples, bone marrow samples, and others. By end user, the market is classified into academic and research centers, hospitals and physician laboratories, pharmaceutical & biotechnology companies, reference laboratories, contract research organizations, and others. Regionally, the companion diagnostics market is analyzed across North America, Europe, Asia-Pacific, Middle East & Africa, and Latin America.
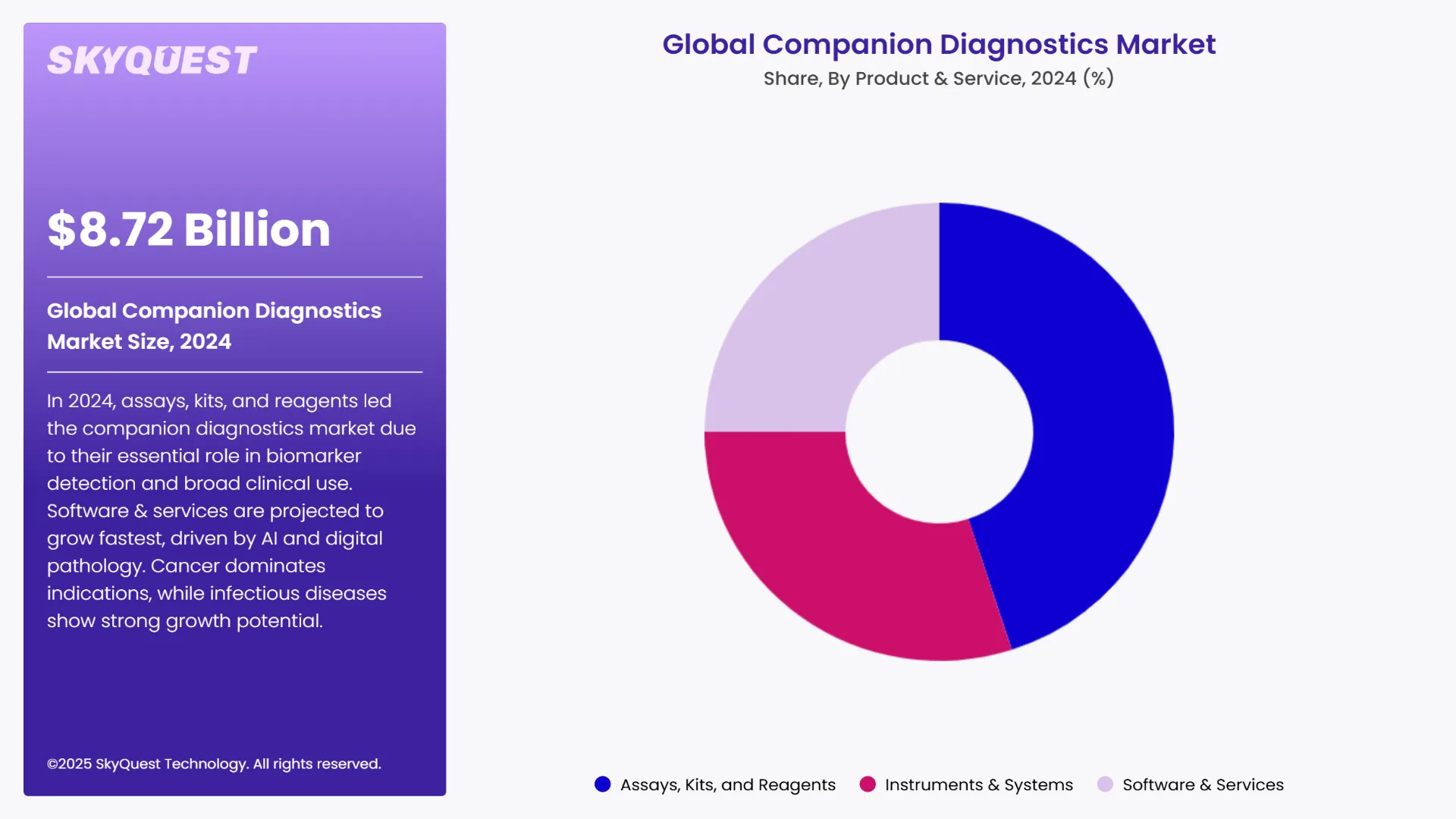
As per the 2024 global companion diagnostics market analysis, the assays, kits, and reagents sub segments led the market by holding the largest share. This is due to the fact that they have very important roles in detecting specific biomarkers that help guide targeted therapies. These are consumables which find use in high volumes across diagnostic workflows. They are widely adopted for oncology and other therapeutic areas. They also find applications in clinical settings. Their huge application is what is making them the most dominant class in the market.
The software & services sub segment is anticipated to grow at the highest CAGR during the forecast period of 2025-2032. This high growth rate can be as a result of the technological advances made recently in the fields of digital pathology, AI, and data analytics. These tools help in better interpretation of diagnostic data. They also help with faster and more accurate decision-making. Other factors such as the growth in demand for integrated diagnostics, remote pathology, and precision medicine are also helping in this market’s growth.
The cancer category in the indication segment is the most dominant in the global companion diagnostics market. This sub segment accounts for the majority of the diagnostics applications. Precision oncology is seeing an upward rise. Here, companion diagnostics are essential for identifying patients eligible for targeted therapies. The high prevalence of different types of cancers like lung cancer, breast cancer, and colorectal cancer, along with the continuous R&D activities and regulatory approvals for oncology-focused CDx makes cancer the largest revenue contributors in the market.
During the forecast period, the infectious diseases’ sub-segment is expected to grow with a notable CAGR. The major driving force of this increase is the legacy left behind by the COVID 19 pandemic and the rising cases of antimicrobial resistance. The demand for precision diagnostics to match patients with antivirals or antibiotics which are most effective is recently a key demand. Recent advancements in technologies of molecular testing, next-generation sequencing, and AI-based analysis are also contributing greatly to its fast growth rate.
To get detailed segments analysis, Request a Free Sample Report
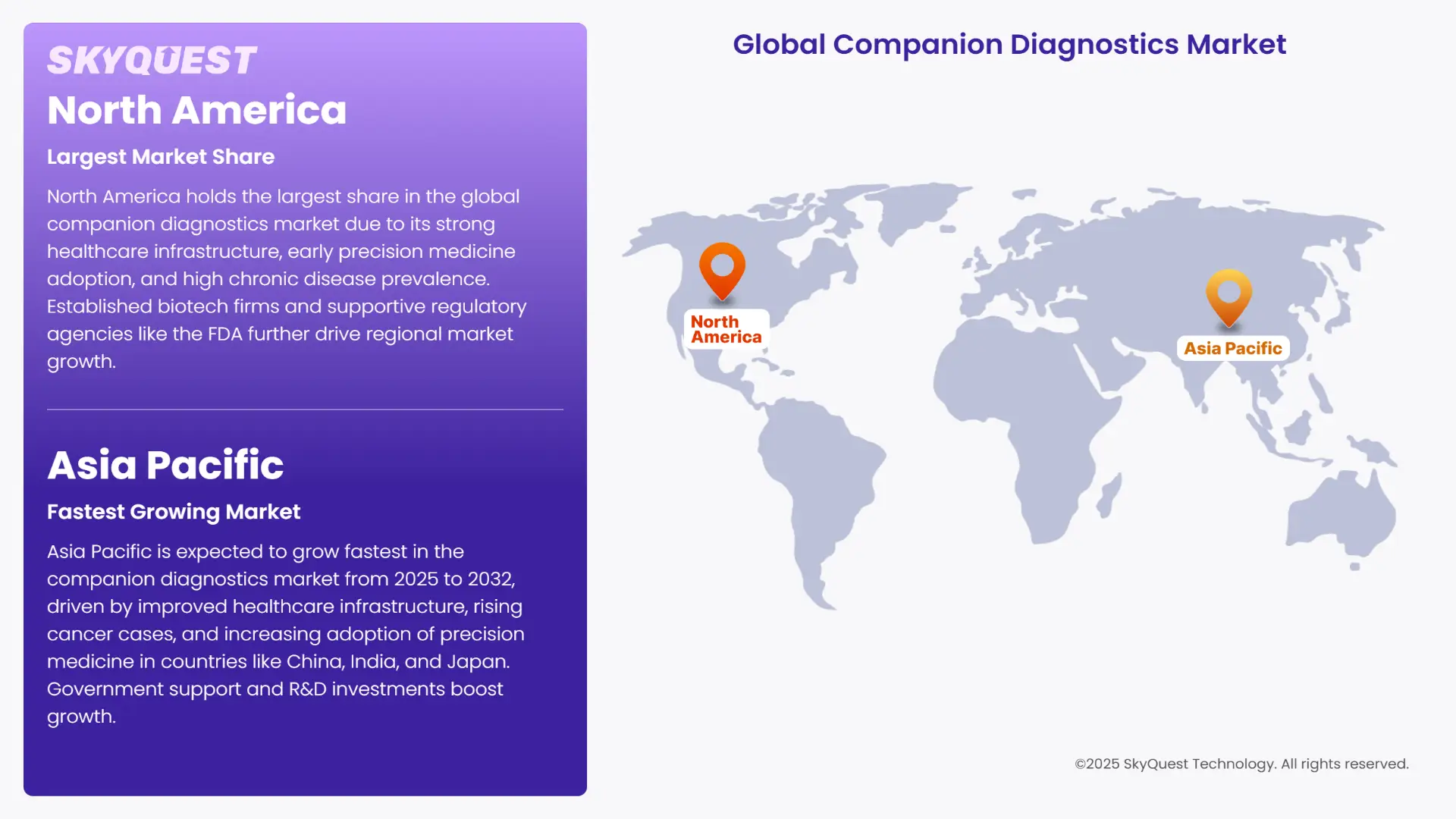
The North America companion diagnostics market share emerges as the most dominant in the world. The region's importance can be attributed to the many established biotechnology, pharmaceutical, and medical device companies in the region. In addition, a strong healthcare infrastructure in the countries of this region, early adoption of precision medicine, and a high prevalence rate of chronic diseases are expected to increase the sector's demand for contract manufacturing independently. The favorable environment provided by the regulatory support of government agencies like the FDA and Canada Drug Agency are also contributing positively towards the growth in companion diagnostics.
U.S. Companion Diagnostics Market
In North America, U.S. leads the companion diagnostics market growth especially due to its well-established and strict regulatory framework, speedy co-development of drug-diagnostic, and strong pharma-biotech partnerships. Many key players in the market such as Agilent Technologies, Thermo Fisher Scientific, Abbott Laboratories, Danaher Corporation, etc. are headquartered here. The FDA’s plays a huge and proactive role in approving CDx with novel therapies which boosts innovation in the country. In November 2024, The FDA approved FoundationOne Liquid CDx. It can be used as a companion diagnostic to identify mNSCLC patients eligible for tepotinib treatment.
Canada Companion Diagnostics Market
Canada in North America is just behind the U.S. The country’s companion diagnostics market growth can also be credited to its strong public healthcare infrastructure, and regulatory pathways which are quite streamlined. In May 2024, Canada’s Drug Agency introduced new improvements to its companion diagnostics reimbursement review process. These amendments include a dedicated Testing Procedure Assessment Report, improved pre-submission templates, and a specialized precision medicine team. This kind of support is highly positive for the market’s growth.
Asia Pacific is predicted to grow at the highest rate in the companion diagnostics market share over the projection period of 2025-2032. The growth in the region is led by the improving healthcare infrastructure and growing burden of cancer in this region. Precision medicine is also seeing higher adoption in the region, especially in countries like China, Japan and India. Governments are actively working on improving access to healthcare to the large pool of patients in the region. The investments on research and development of pharmaceuticals are seeing growth. All these factors contribute to the fast rate at which the companion diagnostics market of Asia Pacific is growing.
China Companion Diagnostics Market
The China companion diagnostics market expansion can be credited to various reasons. This expansion can contribute to a changing market landscape that is seeing advancements in biotechnology and a large patient population. The country’s supporting government initiatives for cancer screening programs also helps the market’s growth. Strategic collaborations between local diagnostic companies and contract research organizations are also accelerating test development. In March 2025, Leica Biosystems and CellCarta partnered with each other to accelerate companion diagnostics development in China. They will utilize Leica’s local R&D and manufacturing capabilities with CellCarta’s advanced biomarker testing services.
Japan Companion Diagnostics Market
Japan’s companion diagnostics market growth is spurred by its adoption of personalized medicine at an early stage and stringent regulatory pathways which has been providing support to drug-diagnostic approvals. In March 2024, Chugai Pharmaceutical received approval in Japan for using the FoundationOne CDx Cancer Genomic Profile as a companion diagnostic for capivasertib in combination with fulvestrant. This is a companion diagnostic for HR-positive, HER2-negative breast cancer with PIK3CA, AKT1, or PTEN alterations. This approval expands the test’s use across 8 cancer types and 27 drugs.
Europe held a significant companion diagnostics market share in 2024 and is likely to continue so during the forecast period of 2025-2032. Governments of countries in Europe provide great regulatory backing to targeted therapies. The European Medicines Agency (EMA) extends support to integrated drug-diagnostic development. This encourages collaboration between pharmaceutical and diagnostic companies. Other factors that support growth of companion diagnostics in the region are the widespread cancer screening programs, increasing clinical trials, and a well-developed healthcare system.
Germany Companion Diagnostics Market
Germany has the largest European companion diagnostics market demand. Germany has a strong pharmaceutical industry and a world-class R&D ecosystem. Germany houses many companies focusing on diagnostics. The country has been making precision medicine in oncology a top priority. The accuracy of diagnostics is quite high in the country. These factors enhance the scope of growth of Germany’s companion diagnostics market.
France Companion Diagnostics Market
The France companion diagnostics market demand is also experiencing steady growth. It is supported by the nation’s healthcare reforms and investments in precision medicine. Programs like France Genomic Medicine 2025 supports CDx development and integration into clinical workflows. This strong regulatory oversight along with increasing physician awareness, makes the France companion diagnostics market a highly opportune market in upcoming years.
To know more about the market opportunities by region and country, click here to
Buy The Complete Report
Increase of Cancer Prevalence to Encourage Market Growth
Regulatory Approval of Innovative Tests for Diverse Indications to Encourage Market Growth
High Development Costs
Technical and Operational Challenges
Request Free Customization of this report to help us to meet your business objectives.
The global companion diagnostic market is a highly competitive business with many established brands, emerging firms, and niche competitors. It has characteristics of an oligopolistic market. One essential element of competition is innovation. The big companies never cut corners when it comes to R&D expenditures since they want to provide cutting-edge, health-conscious flavors, packaging, and tastes. They usually set the pace initially in the industry. Other tactics that these companies use to stay at the top of their games include acquisitions, alliances, and regulatory approvals. Sysmex, for example, expanded its alliance with QIAGEN in August 2024. This was done with the vision to strengthen collaboration in R&D, clinical trials, and global marketing of genetic testing. This relationship makes use of Sysmex’s global lab network and QIAGEN’s expertise in companion diagnostics.
Innovations in Next-Generation Sequencing to Promote Market Growth
Increased Pharma-Diagnostics Collaborations
SkyQuest’s ABIRAW (Advanced Business Intelligence, Research & Analysis Wing) is our Business Information Services team that Collects, Collates, Correlates, and Analyses the Data collected using Primary Exploratory Research backed by robust Secondary Desk research.
As per SkyQuest analysis, the increasing rate of incidence of chronic diseases such as cancers and cases of death due to it is set to drive the global companion diagnostics market outlook to progress. Consequently, assay kits and reagents emerge as the dominant and fastest growing type of product & service for companion diagnostics. North America secures its position as the most dominant region for the market with Asia Pacific following close behind. The market is seeing advancements in technology for diagnosis in the form of integration of bioinformatics and AI tools. The high costs associated with the development of such diagnostic tools and very limited availability of reimbursement policies for patients utilizing companion diagnostics can stand as massive roadblocks. However, the increase in development and regulatory approval of companion diagnostic tools ensure that the market is observing steady growth.
| Report Metric | Details |
|---|---|
| Market size value in 2024 | USD 8.72 Billion |
| Market size value in 2033 | USD 23.8 Billion |
| Growth Rate | 11.8% |
| Base year | 2024 |
| Forecast period | 2026–2033 |
| Forecast Unit (Value) | USD Billion |
| Segments covered |
|
| Regions covered | North America (US, Canada), Europe (Germany, France, United Kingdom, Italy, Spain, Rest of Europe), Asia Pacific (China, India, Japan, Rest of Asia-Pacific), Latin America (Brazil, Rest of Latin America), Middle East & Africa (South Africa, GCC Countries, Rest of MEA) |
| Companies covered |
|
| Customization scope | Free report customization with purchase. Customization includes:-
|
To get a free trial access to our platform which is a one stop solution for all your data requirements for quicker decision making. This platform allows you to compare markets, competitors who are prominent in the market, and mega trends that are influencing the dynamics in the market. Also, get access to detailed SkyQuest exclusive matrix.
Table Of Content
Executive Summary
Market overview
Parent Market Analysis
Market overview
Market size
KEY MARKET INSIGHTS
COVID IMPACT
MARKET DYNAMICS & OUTLOOK
Market Size by Region
KEY COMPANY PROFILES
Methodology
For the Companion Diagnostics Market, our research methodology involved a mixture of primary and secondary data sources. Key steps involved in the research process are listed below:
1. Information Procurement: This stage involved the procurement of Market data or related information via primary and secondary sources. The various secondary sources used included various company websites, annual reports, trade databases, and paid databases such as Hoover's, Bloomberg Business, Factiva, and Avention. Our team did 45 primary interactions Globally which included several stakeholders such as manufacturers, customers, key opinion leaders, etc. Overall, information procurement was one of the most extensive stages in our research process.
2. Information Analysis: This step involved triangulation of data through bottom-up and top-down approaches to estimate and validate the total size and future estimate of the Companion Diagnostics Market.
3. Report Formulation: The final step entailed the placement of data points in appropriate Market spaces in an attempt to deduce viable conclusions.
4. Validation & Publishing: Validation is the most important step in the process. Validation & re-validation via an intricately designed process helped us finalize data points to be used for final calculations. The final Market estimates and forecasts were then aligned and sent to our panel of industry experts for validation of data. Once the validation was done the report was sent to our Quality Assurance team to ensure adherence to style guides, consistency & design.
Analyst Support
Customization Options
With the given market data, our dedicated team of analysts can offer you the following customization options are available for the Companion Diagnostics Market:
Product Analysis: Product matrix, which offers a detailed comparison of the product portfolio of companies.
Regional Analysis: Further analysis of the Companion Diagnostics Market for additional countries.
Competitive Analysis: Detailed analysis and profiling of additional Market players & comparative analysis of competitive products.
Go to Market Strategy: Find the high-growth channels to invest your marketing efforts and increase your customer base.
Innovation Mapping: Identify racial solutions and innovation, connected to deep ecosystems of innovators, start-ups, academics, and strategic partners.
Category Intelligence: Customized intelligence that is relevant to their supply Markets will enable them to make smarter sourcing decisions and improve their category management.
Public Company Transcript Analysis: To improve the investment performance by generating new alpha and making better-informed decisions.
Social Media Listening: To analyze the conversations and trends happening not just around your brand, but around your industry as a whole, and use those insights to make better Marketing decisions.
REQUEST FOR SAMPLE
Global Companion Diagnostics market size was valued at USD 8.72 Billion in 2024 and is poised to grow from USD 9.75 Billion in 2025 to USD 23.8 Billion by 2033, growing at a CAGR of 11.8% in the forecast period (2026–2033).
F. Hoffmann-La Roche Ltd. (Switzerland), Agilent Technologies, Inc. (United States), QIAGEN (Netherlands), Thermo Fisher Scientific Inc. (United States), Abbott Laboratories (United States), Danaher Corporation (United States), Guardant Health, Inc. (United States), Illumina, Inc. (United States), bioMérieux (France), ICON plc (Ireland), Myriad Genetics, Inc. (United States), Sysmex Corporation (Japan), The McClay Foundation (Almac Group) (United Kingdom), ARUP Laboratories (United States), Abnova Corporation (Taiwan), BioGenex (United States), Invivoscribe, Inc. (United States), Laboratory Corporation oof America (ArcherDX, Inc.) (United States), Amoy Diagnostics Co., Ltd. (China), Bio-Techne (Asuragen, Inc.) (United States)
The key driver of the companion diagnostics market is the increasing demand for personalized medicine, which relies on diagnostic tests to identify patients likely to benefit from specific therapies, improving treatment efficacy, safety, and overall patient outcomes.
A key market trend in the companion diagnostics market is the growing integration of molecular diagnostics and biomarker-based testing, enabling more precise patient stratification, targeted therapies, and the development of personalized treatment plans in oncology and other therapeutic areas.
North America accounted for the largest share in the companion diagnostics market, driven by advanced healthcare infrastructure, strong adoption of precision medicine, significant R&D investments, and the presence of leading diagnostic and pharmaceutical companies.
Want to customize this report? This report can be personalized according to your needs. Our analysts and industry experts will work directly with you to understand your requirements and provide you with customized data in a short amount of time. We offer $1000 worth of FREE customization at the time of purchase.
Feedback From Our Clients