
Report ID: UCMIG35I2233

Report ID:
UCMIG35I2233 |
Region:
Global |
Published Date: Upcoming |
Pages:
165
| Tables: 55 | Figures: 60
Pharmaceutical Contract Manufacturing Market is being analyzed by North America, Europe, Asia-Pacific (APAC), Latin America (LATAM), Middle East & Africa (MEA) regions. Key countries including the U.S., Canada, Germany, France, UK, Italy, Spain, China, India, Japan, Brazil, GCC Countries, and South Africa among others were analyzed considering various micro and macro trends.
Our industry expert will work with you to provide you with customized data in a short amount of time.
REQUEST FREE CUSTOMIZATIONThe market for lobal Pharmaceutical Contract Manufacturing was estimated to be valued at US$ XX Mn in 2021.
The lobal Pharmaceutical Contract Manufacturing Market is estimated to grow at a CAGR of XX% by 2028.
The lobal Pharmaceutical Contract Manufacturing Market is segmented on the basis of Region.
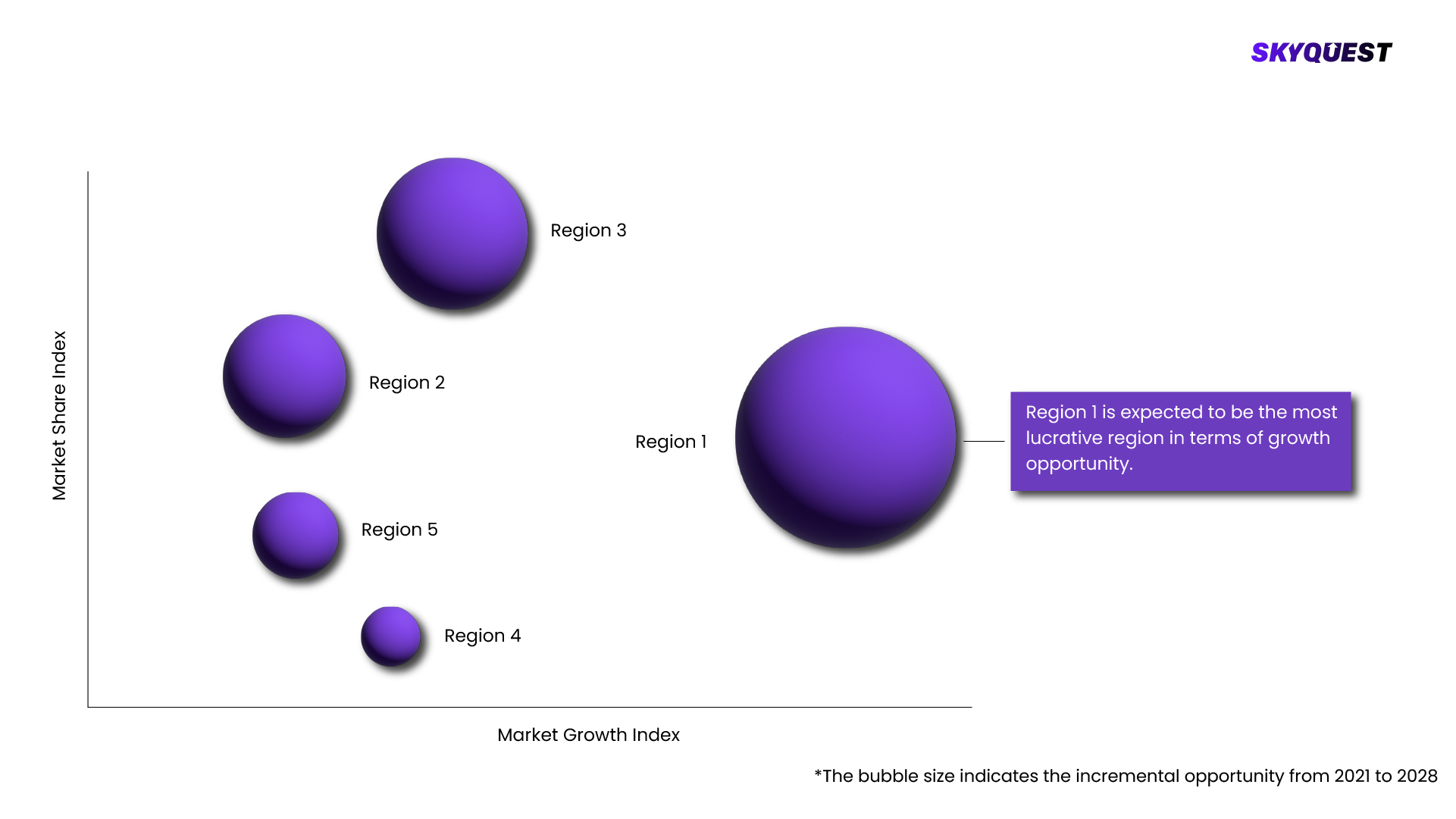
Based on region, the lobal Pharmaceutical Contract Manufacturing Market is segmented into North America, Europe, Asia Pacific, Middle East & Africa and Latin America.
The key players operating in the lobal Pharmaceutical Contract Manufacturing Market are maceutical Contract Manufacturing Market size was valued at USD 17.94 billion in 2021 and is poised to grow from USD 19.0 billion in 2022 to USD 30.06 billion by 2030, growing at a CAGR of 5.9% in the forecast period (2023-2030). , The Pharmaceutical Contract Manufacturing market is witnessing significant growth due to various factors. Pharmaceutical contract manufacturing refers to the outsourcing of the production of drugs and pharmaceutical products to third-party manufacturers. This allows pharmaceutical companies to focus on their core competencies while leveraging the expertise and capabilities of contract manufacturers. , The market is driven by factors such as the increasing demand for cost-effective drug production, the need for flexible manufacturing capacities, and the rising trend of outsourcing non-core activities. , Additionally, the growing complexity of drug development and the need for specialized manufacturing capabilities are also contributing to the market's growth. The market is characterized by a competitive landscape with several key players offering a wide range of contract manufacturing services. , Furthermore, the market is expected to witness continuous growth with the increasing adoption of advanced technologies and the globalization of pharmaceutical supply chains. , One dominant region in the pharmaceutical contract manufacturing market is North America. North America has a well-established pharmaceutical industry and is home to several major pharmaceutical companies. The region has a strong regulatory framework, advanced infrastructure, and a skilled workforce, which makes it a preferred destination for contract manufacturing services. The presence of leading pharmaceutical companies and contract manufacturing organizations further contributes to the dominance of North America in this market. , One of the fastest-growing regions in the pharmaceutical contract manufacturing market is the Asia Pacific. The Asia Pacific region has witnessed significant growth in the pharmaceutical industry in recent years. Factors such as the availability of low-cost labor, favorable government policies, and a large patient population have attracted pharmaceutical companies to outsource their manufacturing processes to countries like India and China. Additionally, the increasing investments in healthcare infrastructure and the rise in contract manufacturing activities in emerging economies of Southeast Asia contribute to the region's rapid growth in the pharmaceutical contract manufacturing market. , Drivers , Increasing Demand For Cost-Effective And Efficient Manufacturing Solutions , One driver of the market is the increasing demand for cost-effective and efficient manufacturing solutions. Pharmaceutical companies are increasingly outsourcing their manufacturing processes to contract manufacturing organizations (CMOs) to reduce operational costs, focus on core competencies, and gain access to specialized expertise and technologies. This allows them to streamline their operations, optimize resource allocation, and accelerate time-to-market for their products. , Restraints , Stringent Regulatory Requirements And Quality Standards Imposed On The Pharmaceutical Industry , On the other hand, a major restraint of the market is the stringent regulatory requirements and quality standards imposed on the pharmaceutical industry. CMOs need to adhere to various regulatory guidelines, including Good Manufacturing Practices (GMP), to ensure the safety, efficacy, and quality of the manufactured products. Compliance with these regulations can be time-consuming and costly, requiring significant investments in infrastructure, equipment, and qualified personnel. Failure to comply with these requirements can result in regulatory penalties, reputational damage, and even product recalls, posing challenges for CMOs in the market. , Pharmaceutical Contract Manufacturing Market Competitive Landscape , The pharmaceutical contract manufacturing market is characterized by a mix of established companies and emerging players. Market participants are focusing on research and development activities to enhance the efficiency and performance of pharmaceutical contract manufacturing. Additionally, strategic collaborations, partnerships, and mergers and acquisitions are prevalent strategies adopted by companies to expand their market presence. The competitive environment is further influenced by factors such as technological advancements, government regulations, and the ability to provide cost-effective and sustainable solutions. , Recent Developments , In January 2023, PharmaCo Solutions announced a strategic partnership with BioGenex Pharmaceuticals to expand its capabilities in biologics manufacturing. The collaboration aims to leverage BioGenex's expertise in biologics development and manufacturing to enhance PharmaCo Solutions' service offerings in the pharmaceutical contract manufacturing market. , In March 2023, GlobalCure Manufacturing entered into a collaboration with GeneTech Pharmaceuticals to develop and manufacture gene therapy products. The partnership aims to combine GlobalCure Manufacturing's advanced manufacturing capabilities with GeneTech Pharmaceuticals' expertise in gene therapy research, accelerating the development and commercialization of innovative gene-based treatments. , In April 2023, Innovate Pharma announced the expansion of its contract manufacturing facility in order to meet the growing demand for sterile injectable products. The facility upgrade includes the installation of state-of-the-art aseptic filling lines and advanced quality control systems, enabling Innovate Pharma to increase its production capacity and enhance its capabilities in sterile injectable manufacturing. , Pharmaceutical Contract Manufacturing Key Market Trends , One key market trend in the market is the increasing demand for outsourcing manufacturing services by pharmaceutical companies. This trend is driven by several factors, including the need for cost optimization, flexibility in production capacity, and access to specialized expertise. Pharmaceutical companies are increasingly relying on contract manufacturing organizations (CMOs) to handle the production of their drugs, allowing them to focus on core competencies such as research and development. , Pharmaceutical Contract Manufacturing Market SkyQuest Analysis , SkyQuest’s ABIRAW (Advanced Business Intelligence, Research & Analysis Wing) is our Business Information Services team that Collects, Collates, Co-relates, and Analyses the Data collected by means of Primary Exploratory Research backed by robust Secondary Desk research. , The pharmaceutical contract market refers to the outsourcing of various pharmaceutical services by pharmaceutical companies to contract manufacturing organizations (CMOs) and contract research organizations (CROs). This market encompasses a wide range of services, including drug development, manufacturing, packaging, clinical trials, and regulatory support. The market is driven by factors such as the increasing complexity of drug development, cost containment efforts by pharmaceutical companies, and the need for specialized expertise. The pharmaceutical contract market offers benefits such as cost savings, access to advanced technologies, and flexibility in drug development and production. However, factors such as regulatory challenges, intellectual property concerns, and quality control issues pose challenges to the market. Overall, the pharmaceutical contract market is witnessing steady growth as pharmaceutical companies leverage external expertise and resources to streamline their operations and bring innovative drugs to market efficiently. , KEY MARKET SEGMENTS , Type , Active Pharmaceutical Ingredient (API) Manufacturing , Finished Dosage Formulation (FDF) Manufacturing , Packaging and Labeling , Others , By Region , North America , Europe , Asia Pacific , Latin America , Middle East & Africa , KEY MARKET PLAYERS , Lonza Group AG - Switzerland , Catalent, Inc. - United States , Patheon N.V. (Thermo Fisher Scientific Inc.) - United States , Recipharm AB - Sweden , Jubilant Life Sciences Ltd. - India , Boehringer Ingelheim GmbH - Germany , Piramal Pharma Solutions - India , Famar Health Care Services - Greece , Siegfried Holding AG - Switzerland , Dr. Reddy's Laboratories Ltd. - India , Aenova Group - Germany , Fareva - France , CordenPharma International - Switzerland , Almac Group - United Kingdom , Metrics Contract Services - United States , WuXi AppTec Inc. - China , NextPharma Technologies Holding Ltd. - United Kingdom , Cambrex Corporation - United States , Biocon Ltd. - India , Hovione - Portugal .
Want to customize this report? This report can be personalized according to your needs. Our analysts and industry experts will work directly with you to understand your requirements and provide you with customized data in a short amount of time. We offer $1000 worth of FREE customization at the time of purchase.

Report ID: UCMIG35I2233